

You don't work out oxidation states by counting the numbers of electrons transferred. If you know how the oxidation state of an element changes during a reaction, you can instantly tell whether it is being oxidised or reduced without having to work in terms of electron-half-equations and electron transfers. Recognising this simple pattern is the single most important thing about the concept of oxidation states. Reduction involves a decrease in oxidation state Oxidation involves an increase in oxidation state Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to an element (a negative oxidation state) to get to its present state. The sulphur has an oxidation state of -2. What if you kept on adding electrons to the element? You can't actually do that with vanadium, but you can with an element like sulphur. You could eventually get back to the element vanadium which would have an oxidation state of zero. The oxidation state of the vanadium is now +5.Įvery time you oxidise the vanadium by removing another electron from it, its oxidation state increases by 1.įairly obviously, if you start adding electrons again the oxidation state will fall. It is also possible to remove a fifth electron to give another ion (easily confused with the one before!). The positive oxidation state is counting the total number of electrons which have had to be removed - starting from the element. Notice that the oxidation state isn't simply counting the charge on the ion (that was true for the first two cases but not for this one). The vanadium is now in an oxidation state of +4. Removal of another electron gives a more unusual looking ion, VO 2+. The vanadium now has an oxidation state of +3. Removal of another electron gives the V 3+ ion: The vanadium is now said to be in an oxidation state of +2. If you think about how these might be produced from vanadium metal, the 2+ ion will be formed by oxidising the metal by removing two electrons: Vanadium forms a number of different ions - for example, V 2+ and V 3+. If you don't know anything about vanadium, it doesn't matter in the slightest. We are going to look at some examples from vanadium chemistry. It would probably be best to read on and come back to these links if you feel you need to. Note: If you aren't sure about either of these things, you might want to look at the pages on redox definitions and electron-half-equations.
Oxidation and reduction in terms of electron transfer However, for the purposes of this introduction, it would be helpful if you knew about: Oxidation states simplify the whole process of working out what is being oxidised and what is being reduced in redox reactions. Oxidation states are straightforward to work out and to use, but it is quite difficult to define what they are in any quick way.Įxplaining what oxidation states (oxidation numbers) are
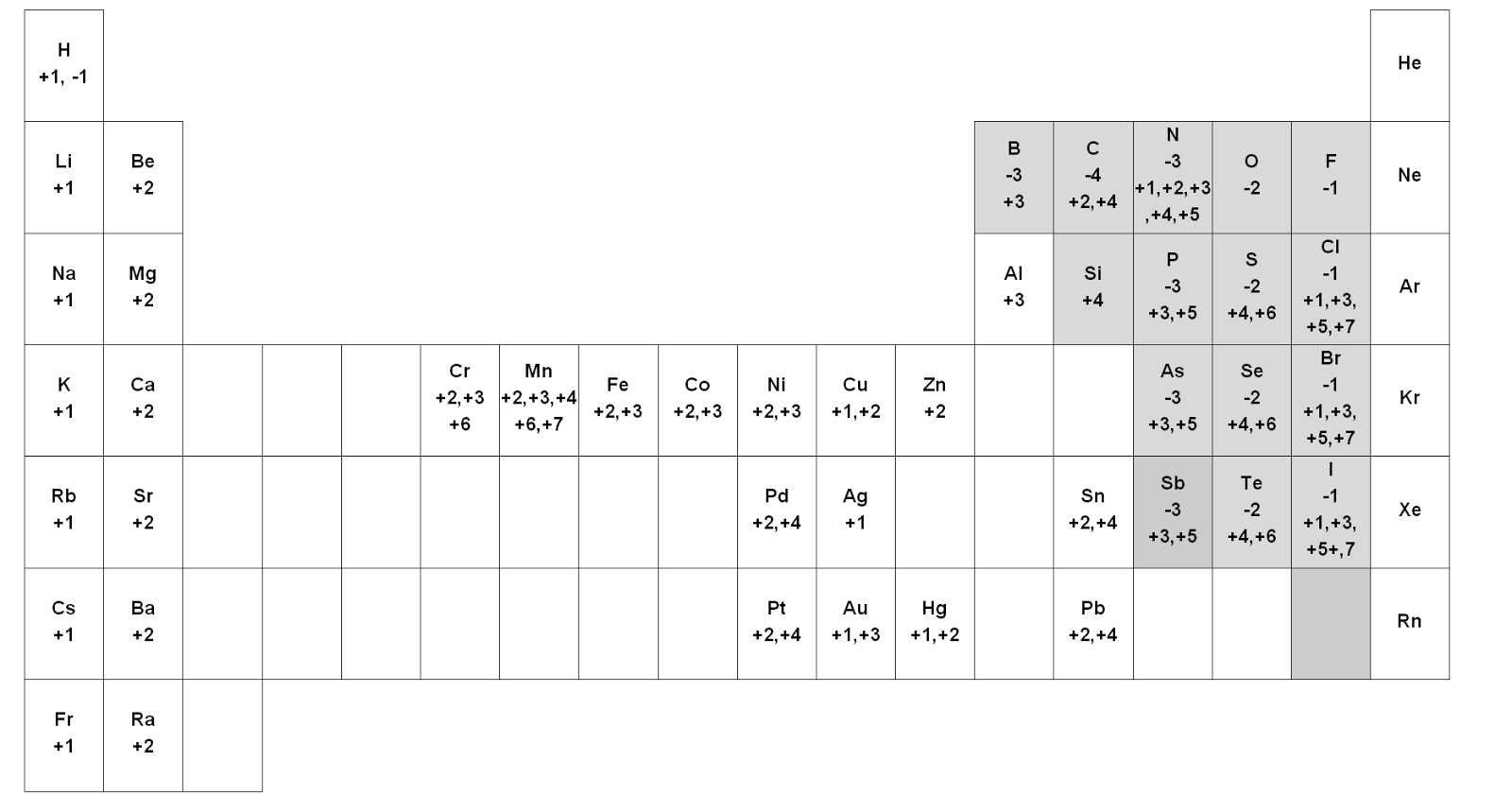
PERIODIC TABLE CHEMISTRY WITH OXIDATION NUMBERS HOW TO
This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them.


 0 kommentar(er)
0 kommentar(er)
